第一部分 基础生化实验
实验一 氨基酸及蛋白质的性质
【实验目的】
1. 加深理解所学有关的蛋白质性质的理论知识
2. 掌握氨基酸和蛋白质常用的定性、定量分析的方法及原理
一、蛋白质呈色反应
蛋白质的呈色反应是指蛋白质所含的某些氨基酸及其特殊结构,在一定条件下可与某些试剂发生了生成有色的物质的反应。
不同蛋白质分子所含的氨基酸残基也是不完全相同,因此所发生的成色反应也不完全一样。另外呈色反应并不是蛋白质的专一反应,某些非蛋白质类物质(含有-CS-NH、-CH2-NH2、-CRH-NH2、-CHOH-CH2NH2等基团的物质)也能发生类似的颜色反应。因此,不能仅仅根据呈色反应的结果为阳性就来判断被测物质一定是蛋白质。
注意:本次实验为定性实验,试剂的量取用滴管完成。
(一)双缩脲反应
【实验原理】
当尿素经加热至180℃左右时,两分子尿素脱去一分子氨,进而缩合成一分子双缩脲。其在碱性条件下双缩脲与铜离子结合成红紫色络合物,此反应称为双缩脲反应。其反应过程如下:
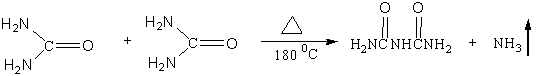

多肽及蛋白质分子结构中均含有许多肽键,其结构与双缩脲分子中的亚酰胺键相同。因此,在碱性条件下与铜离子也能呈现出类似于双缩脲的呈色反应。其反应过程如下:

【试剂】
1. 蛋白质溶液(鸡蛋清用蒸馏水稀释10倍,通过2-3层沙布滤去不容物)
2. 0.1%甘氨酸溶液
3. 0.01%精氨酸溶液
4. 10%NaOH溶液
5. 1%CuSO4溶液
6. 尿素结晶
【实验操作】
1. 双缩脲的制备
取少许尿素结晶 (约火柴头大小)放入干燥的试管中,微火加热至尿素熔解至硬化,刚硬化时立即停止加热,此时双缩脲即已形成。冷却后加10%氢氧化钠溶液约1ml、并震荡,再加入1%硫酸铜溶液2滴,再震荡,观察颜色的变化。
注意:a.在操作过程中试管不能冲向其他人以防止烫伤;
b.控制加热的时间既不能过长也不能过短;
c.加热时火不能太大,防止碳化。
2. 观察现象
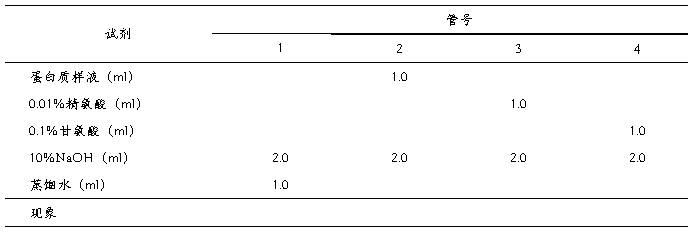
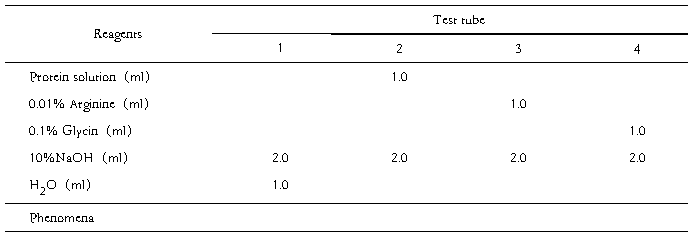
另外取试管4支,按照下表加入各种试剂,观察并解释现象。
表1.
(二)茚三酮反应
【实验原理】
在弱酸条件下(pH5-7),蛋白质或氨基酸与茚三酮共热,可生成蓝紫色缩合物。此反应为一切蛋白质和α—氨基酸所共有(亚氨基酸如脯氨酸和羟脯氨酸产生黄色化合物)。含有氨基的其他化合物亦可发生此反应。
第一步:
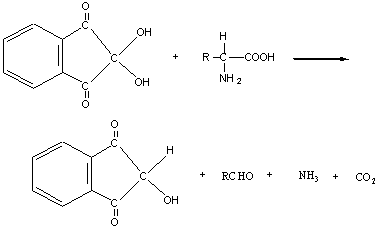
第二步:

【试剂】
1. 蛋白质样液:(与双缩脲反应相同)
2. 0.1%甘氨酸溶液:(与双缩脲反应相同)
3. 0.2%茚三酮溶液
【实验方法】
取试管2支,分别加入蛋白质样液及0.1%甘氨酸溶液1ml,然后各加入茚三酮溶液0.5ml,混匀后于沸水浴中加热数分钟,观察现象,记录结果并解释原因。
(三)蛋白黄反应
【实验原理】
在蛋白质分子中,具有芳香环的氨基酸(如酪氨酸,色氨酸等)残基上的苯环经硝酸作用可生成黄色的硝基化合物,在碱性条件下生成物可转变为桔黄色的硝醌衍生物,反应为:

多数蛋白质分子含有带苯环的氨基酸,所以都会发生黄色反应。苯丙氨酸不易硝化,需加少量浓硫酸后才能够发生黄色反应。
【试剂】
1. 蛋白质溶液(与双缩脲反应相同)
2. 浓硝酸
3. 20%NaOH溶液
4. 0.1%石炭酸溶液
【实验操作】
1. 取1%石炭酸溶液约1ml放在试管内,加浓硝酸5滴,用微火小心加热,观察结果。
2. 取干燥洁净试管1支,加蛋白质样液1ml和浓硝酸5滴,出现沉淀,加热,不必至沸腾,则沉淀变成黄色,待试管冷却后,向两管各加20%NaOH溶液使成碱性,观察颜色变化,记录结果并解释现象。
(四)坂口反应
【实验原理】
蛋白质在碱性溶液中与次氯酸盐(或次溴酸盐)和α-萘酚作用产生红色的产物。这是由于蛋白质分子中精氨酸胍基的特征反应。许多胍的衍生物如胍乙酸、胍基丁胺等也发生此反应。精氨酸是唯一呈正反应的氨基酸,反应灵敏度达1:250000。反应方程式为

生成的氨可被次溴酸钠氧化生成氮。在次溴酸钠缓慢作用下,有色物质继续氧化,引起颜色消失,因此过量的次溴酸钠对反应不利。加入浓尿素,破坏过量的次溴酸钠,能增加颜色的稳定性。此反应可以用来定性鉴定含有精氨酸的蛋白质和定量测定精氨酸的含量。
【试剂】
1. 蛋白质溶液:与双缩尿反应相同
2. 次溴酸钠溶液
3. 10%NaOH溶液
4. 0.2%α-萘酚溶液
5. 0.01%精氨酸溶液
【实验操作】
1. 于试管中加入蛋白质溶液1ml,再加10%NaOH溶液0.5ml,0.2%α-萘酚2滴,混合后再加次溴酸钠溶液2滴,观察现象
2. 取0.01%精氨酸溶液1ml,按上述操作观察现象
蛋白质沉淀反应
蛋白质是亲水胶体,当其稳定因素被破坏或与某些试剂结合成不溶性盐类后,即自溶液中沉淀析出,此现象叫蛋白质的沉淀反应。
(一)蛋白质的盐析作用
【实验原理】
盐析现象是指—般蛋白质在高浓度盐溶液中溶解度下降,故向其溶液中加入中性盐至一定浓度时,蛋白质即自溶液中沉淀析出。盐析作用与两种因素有关:①蛋白质分子被浓盐脱水;②分子所带电荷被中和。
蛋白质的盐析作用是可逆过程,用盐析方法沉淀蛋白质时,较少引起蛋白质变性,经透析或用水稀释时又可溶解。
盐析不同的蛋白质所需中性盐浓度与蛋白质种类及pH有关。分子量大的蛋白质(如球蛋白)比分子量小的(如清蛋白)易于析出。球蛋白在半饱和硫酸铵溶液中即可析出,而清蛋白需在饱和硫酸铵溶液中才能析出。
【试剂】
1. 鸡蛋清的氯化钠溶液(一份鸡蛋清加10份的0.9%氯化钠溶液)
2. 固体硫酸铵
3. 10%氢氧化钠溶液
4. 1%硫酸铜溶液
【实验操作】
1. 取鸡蛋清氯化钠溶液约2ml于试管中,加入硫酸铵粉末,至硫酸铵饱和不再溶解为止,此时溶液颜色为乳白色,用滤纸过滤。
2. 取滤液做双缩脲反应,检查滤液中有无蛋白质存在。
3.蛋白质沉淀用1ml蒸馏水溶解后作双缩脲反应,证明盐析的蛋白质重新溶解于水而未引起变性。
(二)重金属盐类沉淀蛋白质
【实验原理】
溶液pH在蛋白质等电点以上时,重金属盐类(如Pb2+、Cu2+、Hg2+及Ag+等)易与蛋白质结合成不溶性盐而沉淀。
重金属盐类沉淀蛋白质通常比较完全,故常用重金属盐除去液体中的蛋白质。但应注意,在使用某些重金属盐(如硫酸铜或醋酸铅)沉淀蛋白质时,不可过量,否则将引起沉淀再溶解。
【试剂】
1. 蛋白质溶液(与双缩脲反应相同)
2. 5%CuS04溶液
3. 3%AgNO3溶液
【实验操作】
1. 取试管2支各加蛋白质溶液1ml
2. 向各管分别滴加2-3滴加5%CuS04溶液、3%AgNO3溶液,观察各管所生成的沉淀。
3. 在硫酸铜产生蛋白质沉淀的试管中,倒掉大部分沉淀,留少量沉淀,继续加入5%CuS04溶液,观察沉淀的溶解。
(三)有机酸沉淀蛋白质
【实验原理】
生物碱是植物中具有显著生理作用的—类含氮的碱性物质。凡能使生物碱沉淀,或能与生物碱作用产生颜色反应的物质,称为生物碱试剂。如鞣酸、苦味酸和磷钨酸等。当蛋白质溶液pH值低于其等电点时,蛋白质为阳离子,能与生物碱试剂的阴离子结合成性盐而沉淀。溶液中的蛋白亦能被有机酸沉淀,其中以三氯醋酸的作用最为灵敏而且特异,因此广泛的被用于沉淀蛋白质。
【试剂】
1. 蛋白质溶液(与双缩脲反应相同)
2. 10%三氯醋酸溶液
3. 20%水杨磺酸溶液
【实验操作】
1. 取蛋白质溶液1ml于试管中,加数滴三氯醋酸溶液,观察现象;
2. 取蛋白质溶液1ml于试管中,加数滴水杨磺酸溶液,观察现象。
(四)加热沉淀蛋白质
【实验原理】
大多数蛋白质在加热时,由于空间结构被破坏而丧失其稳定性,因此变性凝固。蛋白质的热变性作用与加热时间平行,并随温度的升高而加快。短时间加热可引起凝固。加热时,盐类的存在及溶液酸碱度对蛋白质的凝固有很大影响。处于等电点状态的蛋白质加热时凝固最完全、最迅速。在强酸强碱溶液中,蛋白质分子带有正电荷或负虽加热也不凝固。但溶液中若有中性盐存在,则蛋白质可因加热而凝固。
【试剂】
1. 蛋白质溶液(与双缩脲反应相同)
2. 1%醋酸溶液
3. 10%醋酸溶液
4. 10%氢氧化钠溶液
5. 饱和氯化钠溶液
【实验操作】
取试管4支依下表所示添加试剂。
表2.

加毕混匀,观察各管的情况。然后放入沸水浴中加热10分,注意观察比较各管的沉淀情况。其中2号管溶液接近于蛋白质等电点,最不稳定,最先沉淀;其次是第一管;3号和4号管因在较强的酸或碱下,蛋白质带有大量的电荷,虽然加热也不沉淀。向第三管中加入少许饱和氯化钠溶液,立即出现白色沉淀。
Experiment 1 The Properties of Protein and Amino Acid
【Purposes】
1. Validate the principle about properties of protein and amino acid which we have learned.
2. Master the methods and principles of quantitating and determining the natures of protein and amino acid.
Color Reactions of Protein
Color reactions of protein mean that some chemical bonds of protein or chemical groups of amino acid residues can react with specific reagents to form specific colored substances.
The amino acid residues of different proteins are not quite the same. Therefore, colors of the products are not exactly the same. Color reactions are not the specific reactions of protein and some nonprotein substances (e.g.-CS-NH、-CH2-NH2、-CRH-NH2、-CHOH-CH2NH2) can also have similar color reactions. Consequently we cannot judge protein from the results of the color reactions.
1. Biuret Reaction
【Principle】
Two molecules of carbamide are heated to give a molecule of biuret when the temperature is 180 ℃ and release a molecule of ammonia. Biuret reacts with an alkaline solution of copper cation to give a purple- colored complex. The reaction is called biuret reaction. The process of biuret reaction is as follows:
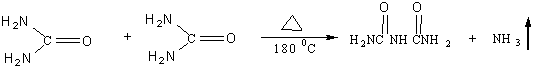

There are many peptide bonds in polypeptides and all of the proteins. The peptide bond is the same as the imido bond of biuret. Therefore protein or poly can react with an alkaline solution of copper cation in a similar way to biuret. The process of reaction is as follows:

【Reagents】
1. Protein solution (Egg white is diluted with distilled water to ten volumes and filtrated by 2--3 layers of gauze)
2. 0.1% Glycinic acid solution
3. 0.01% Argininic acid solution
4. 10% NaOH solution
5. 1% CuSO4 solution
6. Crystal carbamide
【Procedures】
1. Preparation of biuret: Add a little crystal carbamide into a dry test tube, heat by slow fire to liquate, stop heating when it begins to vulcanize, cool and add 1 ml 10% NaOH solution and vulcanize, then add 2 drop of 1% CuSO4 solution, vulcanize again, observe the change of the color.
2. Observing the phenomenon of the other four test tubes, in which the reagents as the following table are added and mixed, then make an explanation.
Table 1.
2. Ninhydrin Reaction
【Principle】
Heating protein or amino acid with ninhydrin in subacid condition can get royal purple condensate. This reaction is the mutual property of all proteins and α- amino acids (amino acid such as proline or hydroxyproline can give yellow condensate). Other compounds containing amino groups also have the ninhydrin reaction.


【Reagents】
1. Protein solution (the same as biuret reaction)
2. 0.1% Glycinic acid solution
3. 0.2% Ninhydrin solution
【Procedures】
Take two test tubes, add four drops of protein solution in one tube and four drops of 0.1% Glycinic acid solution in the other, and then add two drops of ninhydrin solution in each of the tubes. Mix up and heat them in the boiling water bath for several minutes, observe the phenomena and make an explanation.
3. Yellow reaction
【Principle】
Benzene ring in residue of amino acid with aromatic ring in protein molecule (such as tyrosine and tryptophan) can react with nitric acid to give yellow nitro compound, which can change into saffron nitrylchinone derivative in basic condition. The reaction is as follows:
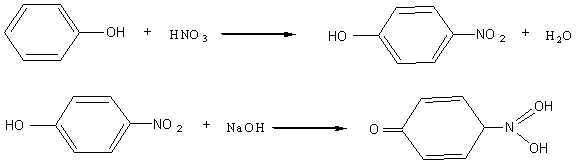
Most proteins have amino acids with aromatic ring, so they have yellow reaction. Phenylalanine is not easy to nitrify, so it is necessary to add in a little vitriol oil.
【Reagents】
1. Protein solution (the same as biuret reaction)
2. Aquafortis.
3. 20% NaOH solution
4. 1% carbolic acid solution
【Procedures】
1. Take a clean and dry test tube, add 1 ml 1% carbolic acid solution and 5 drops of a quafortis, then heat carefully with slow fire and observe the result.
2. Take a clean and dry test tube, add 1 ml protein sample solution and five drops of aquafortis in it, and heat in the boiling water bath for five minutes. Then add 20% NaOH solution in each of the tubes, mix up. Observe color varieties, note down the results and explain them.
4. SaKaguohi Reaction
【Principle】
Protein react with sodium bromate solution and α-naphthol solution in alkali condition to produce red component. This is a special reaction of arginine’s carbamidine, which is useful in quantitating and determining the natures of protein and the amino acid. The reaction is shown as below.

【Reagent】
1. Protein solution (the same as biuret reaction)
2. Sodium bromate solution
3. 10% NaOH solution
4. 0.2%α- naphthol solution
5. 0.01% arginine solution
【Procedures】
1. 1ml of protein solution is added into a test tube, then 0.5 ml of 10%NaOH solution and two drops of 0.2%α- naphthol are added. After mixing, 2 drops of sodium bromate solution are added, and then observe the phenomenon.
2. Add 1 ml of 0.01% arginine solution according to the same procedure above,then observe the phenomenon.
Precipitation Reactions of Protein
Protein is hydrophilic colloid. Protein can be separated out from solution when the stable factors are damaged or when it combines with some agents to become infusibility salts, which is called Precipitation Reaction of Protein.
1. Salting Out of Protein
【Principle】
The solubility of most proteins lowers at a high salt concentration. As neutral salt is added to the protein solution little by little, as the salt concentration is increasing, a point will be reached when the protein comes out of the solution and precipitates. This is called salting out. Two factors relate to salting out.
1. Protein molecules are dehydrated by strong salt solution
2. The charges of protein molecules are neutralized.
Precipitating protein by salting out attributes merely to denaturation of protein. Dialysis or diluted by water can dissolve the precipitation. So salting out is a reversible process.
The neutral salt concentration of precipitating protein by salting out relates to the kind of protein and pH value. Large molecule (such as globulin) is easier to be separated out than small molecule (such as albumin). Half saturated ammonium sulfate solution can precipitate out globulin, whereas saturated ammonium sulfate solution is required to precipitate albumin.
【Reagents】
1. Egg white (egg white with 10 times of 0.9% NaCl solution)
2. Solid ammonium sulfate
3. 10% NaOH solution
4. 1% CuSO4 solution
【Procedures】
1. About 2ml of egg white is placed in a test tube. Add the same volume saturated ammonium sulfate solution, mix round to be uniformity, protein is precipitated out at once. Be standing for several minutes and filtrate by filter paper, the precipitation is egg globulin. If the filtrate is not pellucid, filtrate repeatedly until pellucid.
2. Wash forementioned egg globulin precipitation once by 2ml of half saturated ammonium sulfate solution, and take little precipitation (about match - head size) in lml of distilled water, observe whether it is dissolved, identify by biuret reaction.
3. Place the filtrate in a test tube, add solid ammonium sulfate to be saturated. Observe whether the precipitation is separated out. If there is precipitation, filtrate it. Heat the filtrate and observe whether precipitation is separated out, note down the result and explain the phenomena. The precipitation is egg albumin. We can take little precipitation (about matchhead size) in 1ml of distilled water, observe whether it is dissolved, identify by biuret reaction.
2. Heavy Metal Salts Precipitating Protein
【Principle】
Heavy metal salts (such as Pb2+, Cu2+, Hg2+ and Ag2+ etc.) are easy to combine with protein to give insoluble salts and precipitate when the pH of solution is above the isoelectric point of the protein.
Heavy metal salts precipitating protein is always complete. So heavy metal salts are often used to remove protein in solution. But we must pay attention not to be excessive while using some heavy metal salts (such as CuSO4 or PbAc) to precipitate protein, or else it will cause the precipitation to dissolve.
【Reagents】
1. Protein solution (the same as biuret reaction)
2. 5% CuSO4 solution
3. 3% AgNO3 solution
【Procedures】
① Take two test tubes, and add protein solution 1ml.
② 2-3 drops of 5% CuS04 and 3% AgNO3 are added to two test tubes respectively, then observe the phenomenon.
③ Reserve a bit of precipitation in the test tube with precipitation of protein, then add 5% CuSO4 solution to the tube and observe if the precipitation is dissolved, note down the results.
3. Organic Acid Precipitating Protein
【Principle】
Alkaloid is a kind of nitrogen basic compounds with notable physiological action in plants. All substances that can precipitate alkaloid or react with alkaloid to give color products are called alkaloid reagents, such as tannin, picrinite, phosphowol-framic acid etc. Alkaloid reagents can combine with which proteins are cations to give insoluble salt and precipitate when the pH of solution is under the isoelectric point of the protein. The protein can also be precipitated by organic acid. Trichloroacetic acid is the most sensitive and specific in these acid and is used widely.
【Reagents】
1. Protein solution (the same as biuret reaction);
2. 20% tannin solution;
3. 10% trichloroacetic acid solution.
【Procedures】
① 1ml protein solution is added to a test tube, then add 3 drops of trichloroacetic acid solution, observe and note down the phenomenon.
② 1ml protein solution is added to another test tube, then add 3 drops of tannin solution, observe and note down the phenomenon.
4. Heat Precipitating Protein
【Principle】
Most proteins will be denatured while being heated, because the spatial structure of protein is damaged and protein is not stable.
The thermal denaturation of the protein parallels heating time. It increases with the rise of the temperature. Protein does not solidify in short heating time.
Salts and acid - base. Scale of solution will affect the solidification of protein largely while heating. The solidification of protein is the most complete and quickest at the isoelectric point. Protein has positive or negative electric charge in strong acid or strong base solution. It does not solidify when being heated. But protein can solidify because of being heated while there are neutral salts in solution.
【Reagents】
1. Protein solution (the same as biuret reaction)
2. 1% Acetic acid solution
3. 10% Acetic acid solution
4. 10% NaOH solution
5. Saturated NaC1 solution
【Procedures】
Take four test tubes, and add reagents as the following table.
Table 2.

Shake up after adding, place them in the boiling water bath at the same time, observe and note down the phenomenon and explain them.
