实验十 琼脂糖凝胶电泳鉴定DNA
【实验目的】
通过实验掌握琼脂糖凝胶电泳鉴定DNA的原理与方法。
【实验原理】
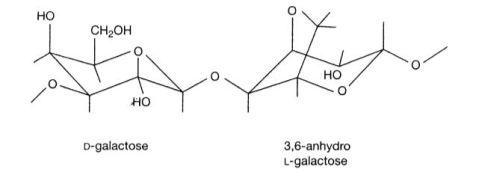
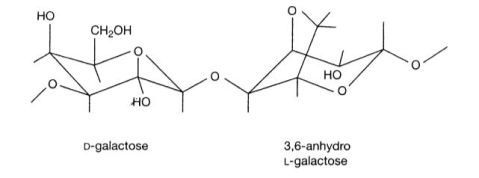
琼脂糖是从海藻中提取出来的一种杂聚多糖,是由D型和L型半乳糖以α-1,3和β-1,4糖苷键相连形成的线状高聚物(如下图所示)。琼脂糖遇冷水膨胀,溶于热水成溶胶,冷却后成为孔径范围从50nm到大于200nm的凝胶。
琼脂糖凝胶电泳是分离、鉴定和纯化DNA片段最为常用的方法之一,这种方法简便易行。而且琼脂糖可以灌制成各种形状、大小和孔径,在不同的装置中进行电泳,如果有必要,还能够从凝胶中回收DNA谱带。
琼脂糖凝胶的分离范围较广,选择不同凝胶浓度和装置,从50个碱基对到几兆不同长度的DNA都可以实现分离。使用电场强度和电泳方向恒定的水平板琼脂糖凝胶电泳的方法,可以很好的分离长度在50-20,000 bp 的DNA片段。
DNA在琼脂糖凝胶中的迁移率受多种因素影响。例如DNA分子的大小;琼脂糖的浓度;所加电压等等。DNA片段越长,泳动速度越慢,而且泳动速度与电场强度成正比。一个给定大小的线性DNA片段,在不同浓度的琼脂糖凝胶中迁移率不同,DNA电泳迁移率的对数与凝胶浓度成线性关系。
用低浓度的荧光染料如溴化乙啶染色后,凝胶中的DNA可以直接被检测出来。紫外灯下可以直接检测到20pg的双链DNA。
【实验材料】
1. 实验器材
水平板电泳槽;灌胶模具及梳齿;电泳仪;55℃水浴;沸水浴;微量移液器
2.实验试剂
(1)DNA样品;DNA标准分子量标记物;琼脂糖;1x电泳缓冲液TBE;6x样品缓冲液
(2)溴化乙锭:水中加入溴化乙啶,搅拌数小时至溶解。将配好的10 mg/ml溴化乙啶溶液装在棕色瓶中,室温保存,使用时稀释至0.5μg/ml。
缓冲溶液配制表
缓冲溶液
TBE
6x样品缓冲液
工作溶液 0.5x 0.045mol/L Tris-硼酸 0.001mol/L EDTA 0.2% 溴酚蓝 50% (w/v) 蔗糖水液 储存溶液(每升) 5x 54 g Tris 碱 27.5g 硼酸 20ml 0.5mol/L EDTA (pH8.0) 储存温度4℃
【实验操作】
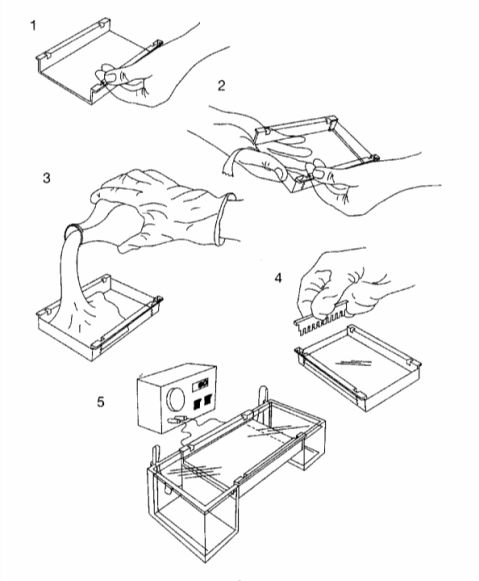
1. 照厂家说明准备好灌胶模具,置于水平面上(实验操作示意图如下)。
2. 配制1%琼脂糖凝胶。取250ml三角瓶,加入100ml TBE缓冲液,称取1克琼脂糖加入缓冲液中,沸水浴加热至完全溶解,然后在55℃水浴中保温。在灌胶模具中插入梳齿,将稍微冷却的凝胶倒入灌胶模具。凝胶厚度3~5mm,避免气泡产生。
3. 室温放置30~45分钟,待凝胶完全凝固后,按照厂家说明将凝胶放入水平板电泳槽。
4. 在电泳槽中加入电泳缓冲液,电泳缓冲液没过胶面1mm,拔下梳齿形成样品池。
5. 取样品与6X样品缓冲液按照比例混合后,用微量移液器缓慢加入样品池中。
6. 盖上电泳槽并且通电,注意电源正负极,确保样品向阳极移动。恒压1-5V/cm(按照两极之间距离计算),至示踪染料溴酚蓝前沿距离凝胶前端1cm时,切断电源。
7. 从电泳槽中取出凝胶,将凝胶置于0.5ug/ml溴化乙啶水溶液中,室温下振摇染色30~45分钟。
8. 回收染色液,自来水冲洗凝胶后,于紫外灯下观察。
【注意事项】
1. 溴化乙啶是一种强诱变剂,并有中度毒性,取用含有这一染料的溶液时务必戴手套。
2. 紫外线对人体,尤其是眼睛有危害性。为减少紫外线照射,必须确保紫外线光源受到遮蔽。
【思考题】
影响琼脂糖凝胶DNA迁移率的因素有那些,分别有怎样的影响?
Experiment 10 Agarose Gel Electrophoresis
【Purpose】
To master the principle and the method of agarose gel electrophoresis and can use it to identify DNA fragments.
【Principle】
Agarose is a linear polymer composed of alternating residues of D- and L-galactose joined by α-(1→3) and β-(1→4) glycosidic linkages (as shown in the following figure). Gelation of agarose
results in a three dimensional mesh of channels whose diameters range from 50 nm to >200 nm.
Agarose Gel Electrophoresis is used to separate, identify, and purify DNA fragments. This technique is simple, rapid to perform. Furthermore, agarose gel can be poured in a variety of shapes, sizes, and porosities and can be run in a number of different configurations. If necessary, these bands of DNA can be recovered from the gel and used for a variety of purposes.
Agarose gel has a wide range of separation DNAs from 50 bp to several megabases in length being separated on agarose gels of various concentrations and configurations. Small DNA fragments (50-20,000 bp) are best resolved in agarose gels run in a horizontal configuration in an electric field of constant strength and direction.
Several factors determine the rate of migration of DNA through agarose gel, including the molecular size of the DNA, the concentration of agarose, the applied voltage and so on. Larger molecules migrate more slowly because of greater frictional drag and because they worm their way through the pores of the gel less efficiently than smaller molecules. At low voltages the rate of migration of linear DNA fragments is proportional to the voltage applied. A linear DNA fragment of a given size migrates at different rates through gels containing different concentrations of agarose. There is a linear relationship between the logarithm of the electrophoresis mobility of the DNA and the gel concentration.
The location of DNA within the gel can be determined directly by staining with low concentrations of fluorescent intercalating dyes, such as ethidium bromide, bands containing as little as 20 pg of double-stranded DNA can be detected by direct examination of the gel in UV.
【Materials】
1. Apparatus:
(1)Clean, dry horizontal electrophoresis apparatus with chamber and come
(2)Boiling water bath
(3)Power supply capable of up to 500v and 200mA
(4)Water bath preset to 55℃
2. Reagent:
(1)DNA sample
(2)DNA size standards
(3)Agarose
(4)Ethidium bromide: Add 1 g of ethidium bromide to 100 ml of H2O. Stir on a magnetic stirrer for several hours to ensure that the dye has dissolved. Wrap the container in aluminum foil or transfer the 10 mg/ml solution to a dark bottle and store at room temperature
Electrophoresis buffer
buffer
TBE
6xGel-loading buffer
Working solution 0.5x 0.045mol/L Tris- boracic acid 0.001mol/L EDTA 0.2% bromophenol blue 50% (w/v) sucrose in H2O Stock solution/liter 5x 54 g of Tris base 27.5 boracic acid 20 ml of 0.5mol/L EDTA (pH8.0) Storage at 4℃
【Procedures】
1. Seal the edges of the plastic tray supplied with the electrophoresis apparatus with tape to form a mode (as shown in the figure following). Set the mode on a horizontal section of the bench.
2. Prepare sufficient 1xTBE to fill the electrophoresis tank and to cast the gel.
3. Prepare 1% (w/v) agarose solution in electrophoresis buffer: Add 1 g powdered agarose to 100 ml 1xTBE in a 250 ml Erlenmeyer flask.
4. Loosely plug the neck of the Erlenmeyer flask with Kimwipes. Heat the slurry in a boiling-water bath until the agarose dissolves.
5. Use insulated gloves to transfer the flask into a water bath at 55℃.
6. While the agarose solution is cooling, position the comb 0.5-1.0 mm above the plate so that a complete well is formed when agarose is added to the mode.
7. Pour the warm agarose solution into the mode, the gel should be between 3 mm and 5 mm thick. Check that no air bubbles are under or between the teeth of the comb.
8. Allow the gel to set completely (30-45 minutes at room temperature), then pour a small amount of electrophoresis buffer on the top of the gel, and carefully remove the comb. Pour off the electrophoresis buffer and carefully remove the tape. Mount the gel in the electrophoresis tank.
9. Add just enough electrophoresis buffers to cover the gel to a depth of ~1 mm.
10. Mix the samples of DNA with 0.2 volumes of the desired 6x gel loading buffer.
11. Slowly load the sample mixture into the slots of the submerged gel using a disposable micropipette. Load size standards into slots on both the right and left sides of the gel.
12. Close the lid of the gel tank and attach the electrical leads so that the DNA will migrate toward the positive anode (red lead). Apply a voltage of 1-5V/cm.
13. Run the gel until the bromophenol blue migrated an appropriate distance through the gel. Turn off the electric current and remove the leads and lid from the gel tank.
14. Stain the gel by immersing in H2O containing ethidium bromide (0.5μg/ml) for 30-45 minutes at room temperature.
15. Examine the gel by UV light.
【Caution】
Ethidium bromide is a powerful mutagen and is toxic. Consult the local institutional safety officer for specific handling and disposal procedures. Avoid breathing the dust. Wear appropriate gloves when working with solutions that contain this dye.
【Questions】

What determine the rate of migration of DNA through agarose gel?
第二篇:琼脂糖凝胶电泳浓度与dna长度的确定
琼脂糖凝胶电泳:胶浓度与DNA长度的关系

- 20##年1月22日

- 1,527 views
- Biology

- 没有评论
分离不同大小的DNA片段所用的最适凝胶浓度是不同的,数据见下表。
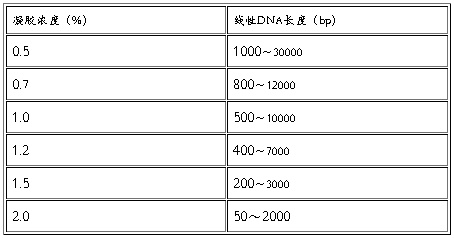
胶回收DNA注意事项

