有 机 化 学 实 验 报 告

实 验 名 称:熔点测定
沸点测定
学 院:化工学院
专 业:化学工程与工艺
班 级:
姓 名 学 号
指 导 教 师:房江华、李颖
日 期
一、 实验目的:
1、了解熔沸点测定的意义;
2、掌握测定熔沸点的操作;
3、认识一些物质的熔沸点。
二、实验原理:
每一个晶体有机物都有一定的熔点(沸点),通过测定熔点(沸点)可以估计有机化合物的纯度;
概念的认识:始熔------样品开始熔化;
熔点距-------开始熔化至完全熔化的温度范围也叫熔点范围和熔程,一般不超过0.5℃;
全熔-----固体样品消失成为透明时。
三、主要试剂及物理性质:


四、实验试剂及仪器:
肉桂酸(纯的)、苯甲酸(纯的)、丙酮(纯的)、乙醇(纯的)、石蜡油、b形管,切口塞子、橡皮圈、熔点毛细管、玻璃管、酒精灯、温度计。
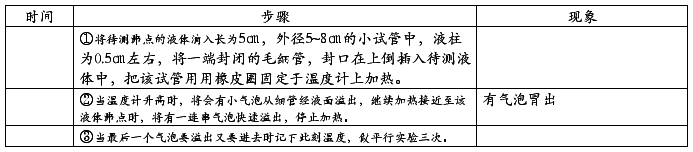
五、实验步骤及现象:
1、熔点的测定:

2、沸点测定:
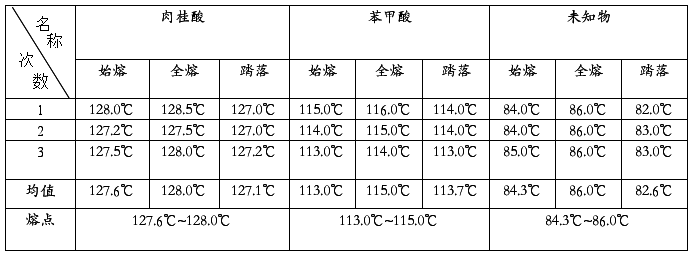
六、数据处理与实验结果:
熔点测定结果
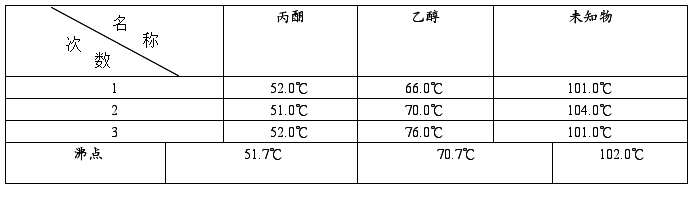
2、沸点测定结果:
七、注意事项:
1、测熔点时:①对长时间加热易分解的,可先将熔点管热至低于样品熔点20℃,再放样品。
②第二次测量时,浴温需降至低于样品熔点20℃再测。
2、测定沸点时:
①每只毛细管只可用一次;
②注意不能让石蜡油碰到橡皮圈;
③热的温度计不能用水洗。
八、实验讨论及误差分析:
①温度计内的煤油柱断裂使读数不准;
②测熔点时,样品熔化温差小,造成读数不精确。
第二篇:有机化学实验报告模式
有机化学实验报告
Experimental Report of Organic Chemistry
班级: 化工121 姓名: 吴才二
同组人: 龙崇燕 实验日期: 2013/9/19
乙醇的蒸馏
Distillation of alcohol
●实验目的 Purpose
1. 了解用蒸馏法分离和纯化物质的原理和方法。
2. 训练蒸馏装置的安装与操作方法,要求整齐、正确。
●实验原理Principle
Distillation has been used of centuries in the purification and separation of liquid organic substances. When the vapor pressure of the liquid being heated equals the atmospheric pressure on the surface of the liquid, it boils. The temperature, at that moment, is called boiling point. Simple distillation is a process that consists of boiling and condensing the vapor to the liquid state.
Every pure organic compound has a fixed boiling point at a certain pressure. Whenever possible, simple distillation is always used to separate liquid mixtures which have boiling points more than about 30℃ apart. But sometimes an organic compound together with other components can form a binary or ternary azeotrop mixture which also has a definite boiling point. So it is difficult to say whether a liquid is a pure organic compound just based on its definite boiling point.
● 实验药品Materials

●实验装置 Apparatus
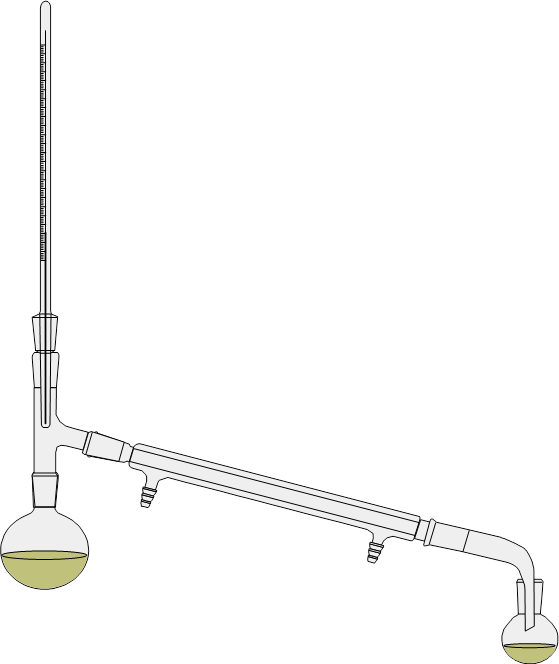
①下图为常见的普通蒸馏装置图,在安装蒸馏装置的时候应该注意哪些问题?
根据蒸馏物的量选择大小合适的蒸馏瓶,一般蒸馏物的体积应占烧瓶容量的1/3~2/3。
温度计通过木塞蒸馏头中央,其水银球上限应和蒸馏头支管的下限在同一水平线上。
蒸馏头的支管和冷凝管相连,用水冷凝时,冷凝管的外套中通水,上端的出水口应向上,可保证内套中充满水,使蒸汽在冷凝管中冷凝为液体。冷凝管的下端和接引管相连。
②标出冷却水进出口。
③ 为什么要加入沸石,加入沸石应注意什么问题?
● 实验过程、步骤 Procedure
1.蒸馏工业酒精:如图安装蒸馏装置。为防止粘牢应在所有玻璃接头上稍稍涂上一些凡士林。注意温度计水银球的上缘应恰好位于蒸馏头侧管下缘所在的水平线上。如果不用标准磨口仪器则支管蒸馏控瓶配以合适的软木塞。
蒸馏装置装好后,将20ml工业酒精倒入50ml的蒸馏烧瓶里,然后往蒸馏烧瓶里放几根毛细管或2~3粒沸石。毛细管和沸石的作用都是为防止暴沸,便沸腾保持平稳。因为当液体在加热时温度有可能上升超过沸点而形成“过热”,此时蒸汽压大大地超过大气压和液柱压力之和,因此上升的气泡增大得非常快,甚至会将液体冲溢出瓶外。这种不正常的沸腾,称“暴沸”。而毛细管和沸石均能产生细小的气泡,形成沸腾中心,使蒸馏能正常进行。
但一旦停止沸腾或中途停止蒸馏,则原有的沸石或毛细管即失效,如再次加热蒸馏前,应补加新的沸石或毛细管。如果事先忘记加入,则必须先移去热源,待加热液体冷至沸点以下方可加入。
接着在冷凝管下口缓缓通入冷水,自上口流出引至水槽中,然后用水浴加热。开始时温度可上升稍快些,待蒸馏瓶内液体沸腾时应控制加热,使液体流出速度为每秒钟1~2滴为宜。当温度趋于稳定时,另换接收器收集,记录此时的温度,继续蒸馏,当温度计读数突然下降或蒸馏瓶内只剩下很少液体时,即可停止加热,并记下此时温度。这两个温度即为乙醇的沸程。量取所收集馏份的体积,并计算回收率。注意不要蒸干,以免蒸馏瓶破裂或发生其他意外事故。
蒸馏完毕,应先停火,然后停止通水,拆下仪器,拆除仪器的程序和装置仪器的程序相反,即依次取下接收器,接引管,冷凝器和蒸馏瓶。
● 实验记录及数据处理 Data and Analysis
实验现象及记录:
是否有异常现象:
相关数据与讨论:
● 思考题 Questions
1. 蒸馏提纯时,一般应记录几个区段的馏分? 分三个区段
为什么?因为
Key Notes:
① Adding boiling chips can produce smooth bubbling and prevent boil-over or bumping of the liquid. Avoid adding them to a heated liquid. If distilling is started after heating is stopped.
②Introduced tap water from a horse into the lower condenser nipple and have it exit out of the upper nipple.
③Don’t distill to dryness to prevent any experimental accidents.
有机化学实验报告
Experimental Report of Organic Chemistry
班级: 化工121 姓名: 吴才二
同组人: 龙崇燕 实验日期:
溴乙烷的制备
Preparation of Ethyl bromide
●实验目的 Purpose
1. 学习从醇制备溴代烷的原理和方法。
2. 进一步巩固分液漏斗的使用及蒸馏操作。
●实验原理 Principle
Ethyl bromide can be easily prepared by allowing alchol to react with sodium bromide and concentrated sulfuric acid.
But pay attentiong, concentrated sulfuric acid can also catalyze intermolecular or inmolecular dehydration of alchol to produce diethyl ether or ethylene.
主反应:NaBr+H2SO4
副反应
● 实验药品 Materials

● 实验过程、步骤 Procedure
●实验装置 Apparatus
● 实验记录及数据处理 Experiment Record and Data Analysis
实验现象及记录:
是否有异常现象:
相关数据与讨论:
● 思考题 Questions
1. 溴乙烷沸点很低,很容易挥发。在实验中应该注意那些问题?
对于实验过程中出现倒吸现象,应该怎么处理?
你有什么好的建议:
2. 本实验中反应初期为什么采用小火?
3.本实验中硫酸起了多种作用,请分别说明(并分别配以方程式):
4.溴乙烷蒸馏之前如果除水不尽,会造成什么影响?
5.本次实验中安装的两次蒸馏装置有什么不同,分别起什么作用?
Key Notes
1) Add water to the flask before adding sulfuric acid. Add water to prevent bubbling over.
It is important to shake the flask and cool down the mixture.
2) Be careful to save the organic layer.
3) Concentrated sulfuric acid is used to get rid of unreacted alchol, diethyl ether and water. ethyl bromide and alchol can form a binary azeotrope mixture, boiling point 37℃
4)Dry the crude ethyl bromide using a small amount of anhydrous calcium chloride until the liquid is clear.
有机化学实验报告
Experimental Report of Organic Chemistry
班级: 姓名:
同组人: 实验日期:
环己酮的制备 (6 学时)
Preparation of Cyclohexanoe
●实验目的 Purpose
1.
2.
●实验原理Principle
Cyclohexanoe is usually prepared by oxidation of cyclohexanol using chromic acid as oxidizing agent.
主反应
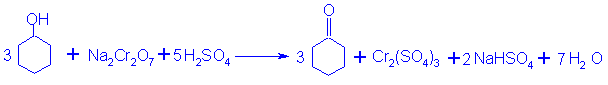
副反应
水蒸气蒸馏的原理:
Steam distillation, one of the immiscible phase being water, is one of the most commonly used method of separating and purifying liguids. In this method steam is passed into the immiscible and volatile liquid in the distillation flask, and then the water and organic compound co-distill, condense in the condenser, and are collected in the normal way.
According to the law of partial pressure, the totle vapor pressure above the mixture is the sum of the partial pressures of the pure individual components. Therefor, when the sum of the individual vapor pressures is equal to the atmospheric pressure,the mixture will start boiling at a lower temperature(boiling point) than either of the individual components. That means that the organic compound will distill out below its normol boiling point.
水蒸气蒸馏常用于下列情况:①除去挥发的固体有机物质(普通蒸馏时冷凝管易被堵塞);
②
③
● 实验药品 Materials

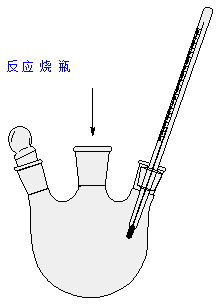 ●实验装置 Apparatus
●实验装置 Apparatus
反应装置
反应烧瓶中插入温度计的作用是什么?
答:
简化水蒸气蒸馏装置
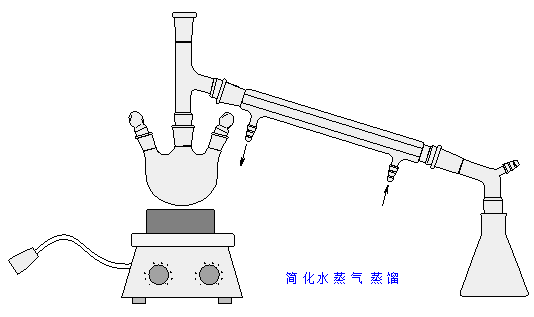
反应装置中是否应该插入温度计?
答:
● 实验过程、步骤 Procedure
● 实验记录及数据处理 Experiment Record and Data Analysis
实验现象及记录:
是否有异常现象:
相关数据与讨论:
● 思考题 Questions
1. 氧化反应进行时,为什么要待反应物橙红色完全消失后再加入下一批重铬酸钠?
2. 整个氧化反应过程中,温度应控制在什么范围
原因:
3. 蒸馏过程中应使用 冷凝管,原因
4. 馏出液中加盐的作用是
Key Notes:
1) In order to remove the excess acid, saturated sodium bisulfite solution should be added.
2) The solubility of cyclohexanone in water is 2.4g/100ml at 31℃, in order to aid in layer separation, 4~5 g of sodium chloride is added.
有机化学实验报告
Experimental Report of Organic Chemistry
班级: 姓名:
同组人: 实验日期:
乙酸正丁酯的制备
Preparation of n-Butyl Acetate
●实验目的 Purpose
1.
2.
●实验原理 Principle
n-Butyl acetate can be prepared with acetic acid and n-butyl alcohol by a single step reaction using a catalytic amount of concentrated sulfuric acid.
主反应
副反应
● 实验药品 Materials

● 实验过程、步骤 Procedure
●实验装置 Apparatus
补齐主反应装置图

补齐蒸馏装置图:
● 实验记录及数据处理 Data and Analysis
实验现象及记录:
是否有异常现象:
相关数据与讨论:
● 思考题 Questions
1. 本实验采用分水器的原理是什么?
分水器操作的方法是:
2. 本实验中分水器上层有机物的成分是什么?
3. 本实验中不能用氯化钙代替硫酸镁作干燥剂的原因是:
4. 使用分水器与不使用分水器的区别是:
Key Notes
1) The concentrated sulfuric acid is a catalyst in this reaction,but the amount of the sulfuric acid is very important in the reaction. You must not use too much, just a catalytic amount.
2) The sulfuric acid should be added dropwise or the experiment will fail.
3) There are two ways to judge whether the reaction has finished: ⑴ there are no more water drops sinking; ⑵ compare the amount of the water from the separator with the theoretical amount of the water produced in the reaction.
