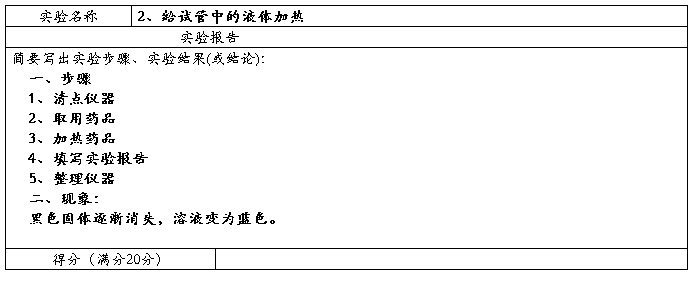
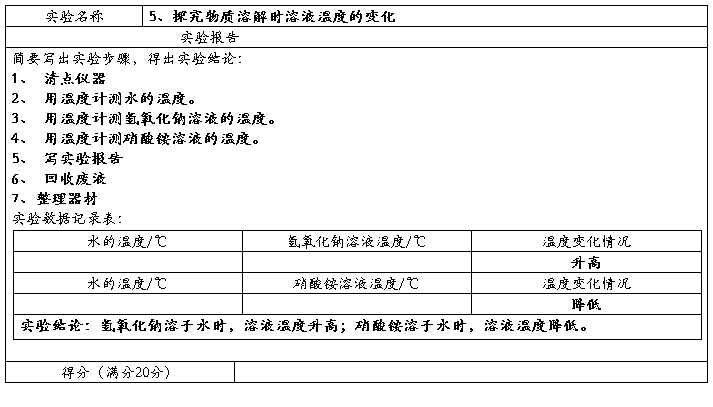
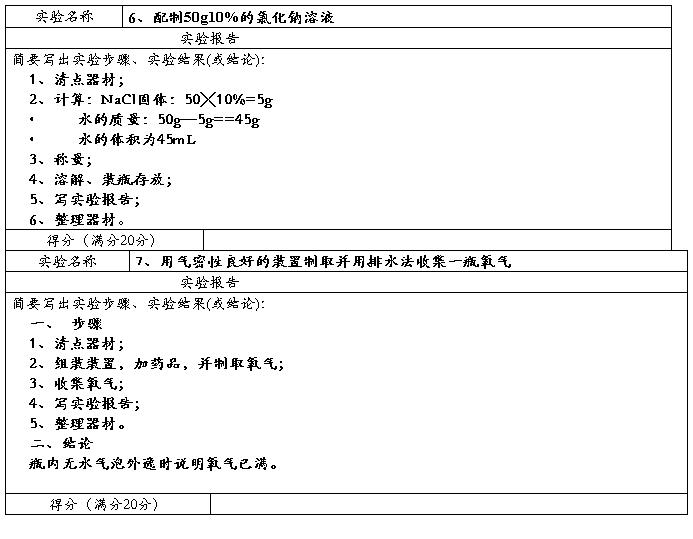
Preparation of ethyl acetate
First, thepurpose of the experiment:
1、 Learn from the general principles of organic synthetic esters and methods
2、 Master distillation, extraction, drying and other experimental techniques and its application in a specific experiment
Second, theexperimental principle:
Main reaction:
CH3COOH+CH3CH2OH=CH3COOCH2CH3+H2O
Conditions: heating to 120 to 125 °C in concentrated sulfuric acid catalyzed
Side effects:
浓H2SO4
CH3CH2OH--------->CH2=CH2+H2O
170度
…… …… 余下全文

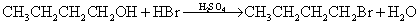
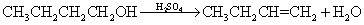 ;
;